Global Stroke Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
33.00 Billion
USD
58.93 Billion
2024
2032
USD
33.00 Billion
USD
58.93 Billion
2024
2032
| 2025 –2032 | |
| USD 33.00 Billion | |
| USD 58.93 Billion | |
|
|
|
|
Segmentación del mercado global de accidentes cerebrovasculares, tipo (accidente cerebrovascular isquémico, accidente isquémico transitorio [AIT] y accidente cerebrovascular hemorrágico ), diagnóstico y tratamiento (diagnóstico y tratamiento), género (femenino y masculino), usuario final (hospitales y clínicas, clínicas especializadas, centros de cirugía ambulatoria, atención domiciliaria, laboratorios y otros), canal de distribución (directo, minorista y en línea): tendencias de la industria y pronóstico hasta 2032.
Análisis del mercado de accidentes cerebrovasculares
El mercado global del ictus es un sector en rápido crecimiento dentro de la industria de la salud, centrado en productos y servicios destinados a la prevención, el diagnóstico, el tratamiento y la rehabilitación de pacientes con ictus. Los componentes clave de este mercado incluyen productos farmacéuticos (como trombolíticos, antiagregantes plaquetarios y anticoagulantes), dispositivos médicos (como stents vasculares y dispositivos neuroprotectores) y equipos de rehabilitación (incluyendo herramientas de fisioterapia y logopedia). Este crecimiento se debe a factores como la mayor concienciación sobre la prevención del ictus, los avances en la tecnología médica y el aumento de la población geriátrica, más susceptible a sufrirlo. La creciente prevalencia de enfermedades no transmisibles, en particular la hipertensión y la diabetes, contribuye a la demanda de una atención eficaz para el ictus.
Tamaño del mercado de accidentes cerebrovasculares
Se espera que el mercado global de accidentes cerebrovasculares alcance los USD 58,93 mil millones para 2032 desde USD 33,00 mil millones en 2024, creciendo a una CAGR del 7.8% en el período de pronóstico de 2025 a 2032. Además de la información sobre escenarios de mercado como el valor de mercado, la tasa de crecimiento, la segmentación, la cobertura geográfica y los principales actores, los informes de mercado seleccionados por Data Bridge Market Research también incluyen un análisis profundo de expertos, epidemiología de pacientes, análisis de cartera, análisis de precios y marco regulatorio.
Tendencias del mercado de accidentes cerebrovasculares
Adopción creciente de tecnologías avanzadas de neuroimagen y soluciones de telemedicina para el diagnóstico y el tratamiento del ictus.
Una tendencia notable en el mercado global del ictus es la creciente adopción de tecnologías avanzadas de neuroimagen y soluciones de telemedicina para su diagnóstico y tratamiento. A medida que aumenta la conciencia sobre la importancia crucial de la intervención rápida en el tratamiento del ictus, se observa una tendencia significativa hacia el uso de técnicas de imagen sofisticadas, como la resonancia magnética y la tomografía computarizada, que permiten un diagnóstico más rápido y preciso de los diferentes tipos de ictus. Además, la telemedicina se está convirtiendo en un elemento fundamental para brindar consultas oportunas y monitorización remota, especialmente en zonas rurales o desatendidas, lo que permite a los profesionales sanitarios evaluar a los pacientes e iniciar tratamientos con prontitud. Esta tendencia no solo mejora los resultados de los pacientes, sino que también impulsa la innovación y la inversión en el continuo de atención del ictus.
Alcance del informe y segmentación del mercado de accidentes cerebrovasculares
|
Atributos |
Perspectivas clave del mercado de accidentes cerebrovasculares |
|
Segmentos cubiertos |
|
|
Países cubiertos |
EE. UU., Canadá, México, Alemania, Francia, Reino Unido, Países Bajos, Suiza, Rusia, Italia, España, Turquía, Austria, Polonia, Noruega, Irlanda, Resto de Europa, China, Japón, India, Corea del Sur, Singapur, Malasia, Australia, Tailandia, Indonesia, Filipinas, Vietnam, Taiwán, Resto de Asia-Pacífico, Arabia Saudita, Emiratos Árabes Unidos, Sudáfrica, Egipto, Israel, Kuwait, Resto de Oriente Medio y África, Brasil, Argentina, Chile, Perú, Resto de Sudamérica |
|
Actores clave del mercado |
Bristol-Myers Squibb Company (EE. UU.), Boehringer Ingelheim International GmbH (Alemania), F. Hoffmann-La Roche Ltd (Suiza), DAIICHI SANKYO COMPANY, LIMITED (Japón), Sanofi (Francia), Johnson & Johnson Services, Inc. (EE. UU.), Bayer AG (Alemania), Sandoz AG (Suiza), Pfizer Inc. (EE. UU.), Medtronic (Irlanda), Abbott (EE. UU.), Viatris Inc. (EE. UU.), AstraZeneca (Reino Unido), Penumbra, Inc. (EE. UU.), GLENMARK PHARMACEUTICALS LTD (India), Fresenius SE & Co. KGaA (Alemania), Teva Pharmaceuticals USA, Inc. (Israel), Lupin (India) y Amneal Pharmaceuticals LLC (EE. UU.), entre otros. |
|
Oportunidades de mercado |
|
|
Conjuntos de información de datos de valor añadido |
Además de los conocimientos sobre escenarios de mercado, como el valor de mercado, la tasa de crecimiento, la segmentación, la cobertura geográfica y los principales actores, los informes de mercado seleccionados por Data Bridge Market Research también incluyen un análisis profundo de expertos, epidemiología de pacientes, análisis de la cartera de productos, análisis de precios y marco regulatorio. |
Definición del mercado de accidentes cerebrovasculares
El mercado global del ictus abarca los diversos productos, servicios y tecnologías involucrados en la prevención, el diagnóstico, el tratamiento y la rehabilitación de pacientes con ictus en todo el mundo. Esto incluye una gama de dispositivos médicos, productos farmacéuticos, equipos de imagenología y soluciones terapéuticas destinadas a abordar las complejidades de la atención del ictus. El mercado se ve impulsado por la creciente incidencia del ictus debido al envejecimiento de la población, factores relacionados con el estilo de vida y una mayor concienciación sobre los síntomas y las opciones de tratamiento. Además, los avances en las tecnologías sanitarias y la telemedicina están transformando el panorama, facilitando una mejor gestión del paciente y mejorando los resultados en la atención del ictus en diversos grupos demográficos y entornos sanitarios.
Dinámica del mercado de accidentes cerebrovasculares
Conductores
- La creciente incidencia de accidentes cerebrovasculares impulsa la demanda de tratamientos
La creciente incidencia de accidentes cerebrovasculares es un factor determinante en el mercado mundial de accidentes cerebrovasculares, que influye tanto en la demanda de tratamiento como en la infraestructura sanitaria. Los accidentes cerebrovasculares, una de las principales causas de discapacidad y muerte en todo el mundo, son cada vez más comunes debido a diversos factores de riesgo, como el envejecimiento de la población, el sedentarismo, la hipertensión arterial, la diabetes, el tabaquismo y la mala alimentación. A medida que aumenta la esperanza de vida y la población envejece, también ha aumentado la prevalencia de afecciones que contribuyen al accidente cerebrovascular, como la hipertensión y la fibrilación auricular, lo que resulta en un mayor número de personas que sufren accidentes cerebrovasculares y requieren atención médica inmediata y rehabilitación a largo plazo.
Por ejemplo,
En mayo de 2023, según un artículo publicado en eClinicalMedicine, el ictus era la segunda causa principal de muerte y la tercera causa principal de discapacidad a nivel mundial. En los últimos 30 años, se ha observado un aumento en el número absoluto de ictus incidentes (70%) y prevalentes (85%), así como en el de muertes (43%) por ictus.
- Número creciente de pacientes con hipertensión y enfermedades coronarias
La hipertensión, comúnmente conocida como presión arterial alta, es una afección médica que se caracteriza por una mayor presión arterial contra las paredes de las arterias. Se define típicamente por una lectura de presión arterial de 130/80 mm Hg o superior y puede clasificarse como esencial (primaria) o secundaria, según su causa subyacente. La hipertensión prolongada puede provocar diversos problemas de salud graves, siendo uno de los más importantes la enfermedad coronaria. La enfermedad coronaria, también conocida como enfermedad de las arterias coronarias, resulta de la acumulación gradual de depósitos de grasa (ateroesclerosis) en las arterias coronarias, que suministran oxígeno y nutrientes al músculo cardíaco. A medida que estas arterias se estrechan o se obstruyen, el flujo sanguíneo al corazón se reduce, lo que provoca dolor torácico (angina) y, en casos graves, infartos.
Por ejemplo,
En septiembre de 2023, según un artículo publicado en la Revista de la Organización Panamericana de la Salud, la hipertensión, a menudo asintomática, contribuye significativamente a las enfermedades cardiovasculares, que son la principal causa de muerte. Factores como el envejecimiento, la obesidad y los malos hábitos de vida están impulsando el aumento de la prevalencia, lo que exige tratamientos eficaces.
Oportunidades
- Desarrollo de terapias avanzadas para accidentes cerebrovasculares
El desarrollo de terapias avanzadas representa una oportunidad significativa para el mercado global del ictus, al abordar las importantes necesidades insatisfechas en la atención del ictus. Los tratamientos actuales, centrados principalmente en restablecer el flujo sanguíneo mediante trombólisis o trombectomía, son eficaces solo en un período limitado y no abordan el daño neuronal subyacente. Las terapias avanzadas, como los agentes neuroprotectores, las terapias celulares y los sistemas de administración dirigida de fármacos, prometen mitigar este daño, promover la reparación neuronal y mejorar los resultados funcionales a largo plazo de los pacientes con ictus. Esto conducirá a una disminución de la discapacidad, una reducción de los costos de atención médica asociados con la atención a largo plazo y una mejor calidad de vida para los sobrevivientes, ampliando así el potencial del mercado al atraer inversión e impulsar la demanda de tratamientos más efectivos.
Por ejemplo,
En abril de 2022, según un artículo publicado por la American Heart Association Journals, el tratamiento del ictus isquémico agudo continúa avanzando. La tenecteplasa se ha evaluado como un fármaco trombolítico alternativo y la evidencia sugiere que es al menos tan eficaz como la alteplasa y puede lisar coágulos de grandes vasos con mayor eficacia. La terapia endovascular con trombectomía mecánica ha demostrado ser beneficiosa hasta 24 horas después del inicio del ictus en pacientes cuidadosamente seleccionados con oclusiones proximales de grandes vasos.
- Expansión de los servicios de rehabilitación de accidentes cerebrovasculares
La expansión de los servicios de rehabilitación de accidentes cerebrovasculares representa una oportunidad sustancial para el mercado global de accidentes cerebrovasculares, al abordar la creciente necesidad de programas de recuperación y rehabilitación más efectivos. Actualmente, quienes sobreviven a accidentes cerebrovasculares a menudo enfrentan dificultades significativas para recuperar las funciones motoras y cognitivas perdidas, lo que resulta en hospitalizaciones prolongadas, mayores costos médicos y una menor calidad de vida. A medida que la población mundial envejece y las tasas de incidencia de accidentes cerebrovasculares aumentan, existe una necesidad apremiante de servicios de rehabilitación mejorados que satisfagan las necesidades individuales de los sobrevivientes. Al expandir los servicios de rehabilitación de accidentes cerebrovasculares, los proveedores de atención médica y las aseguradoras pueden abordar la demanda insatisfecha de atención integral y personalizada, lo que se traduce en mejores resultados para los pacientes, menores costos de atención médica y mayor satisfacción del paciente.
Por ejemplo,
En abril de 2023, según un artículo publicado por el MDPI, los países desarrollados se esfuerzan por brindar rehabilitación a los pacientes con ictus. La rehabilitación física puede reducir o prevenir las complicaciones conocidas en pacientes con ictus, a la vez que mejora su calidad de vida. Los terapeutas eligen las intervenciones según las discapacidades, las limitaciones de la actividad y los objetivos de recuperación.
Restricciones/Desafíos
- Alto costo del diagnóstico
Las enfermedades cardíacas y los accidentes cerebrovasculares son uno de los principales factores del aumento de la tasa de mortalidad a nivel mundial a lo largo de los años. El accidente cerebrovascular puede clasificarse entre las enfermedades crónicas más costosas. Más de 868,000 estadounidenses mueren cada año por enfermedades cardíacas o accidentes cerebrovasculares, lo que representa un tercio de todas las muertes. Con el aumento de la incidencia de accidentes cerebrovasculares, el costo del diagnóstico y el tratamiento ha aumentado con los años, lo cual constituye el principal factor limitante.
La mayoría de los pacientes no solo padecen discapacidades de por vida que afectan su sustento, sino que también tienen un enorme impacto económico en la sociedad. El costo del diagnóstico también ha aumentado con el avance tecnológico.
Por ejemplo,
Según la Agencia para la Investigación y la Calidad de la Atención Médica, la internación hospitalaria promedio por accidente cerebrovascular isquémico (que incluye diagnóstico y estadía) es de 5,6 días a USD 9.100 por estadía, y por accidente cerebrovascular hemorrágico, es de 8,4 días a USD 19.500 por estadía.
- Aumento del retiro de productos
Los profesionales utilizan una amplia gama de dispositivos de diagnóstico para el ictus para realizar diferentes procedimientos en pacientes de distintas edades. Por lo tanto, los efectos secundarios y las complicaciones asociados con el uso de estos dispositivos pueden causar graves daños a los pacientes.
Además, estos dispositivos y productos de diagnóstico son muy costosos y muy riesgosos, y una posible falla podría tener consecuencias graves para el paciente. Por lo tanto, están estrictamente regulados y se retiran del mercado para garantizar la seguridad de los pacientes.
Por ejemplo,
El sistema de escáner CT multicorte NeuViz 64 de Neusoft Medical Systems Co., Ltd., que es un sistema de escáner CT multicorte utilizado como sistema de rayos X de tomografía computarizada de cuerpo completo que cuenta con un tubo de rayos X que gira de manera continua y un conjunto de detectores, ha sido retirado del mercado por la FDA debido a un error de software en el sistema.
Este informe de mercado proporciona detalles sobre los últimos desarrollos, regulaciones comerciales, análisis de importación y exportación, análisis de producción, optimización de la cadena de valor, cuota de mercado, impacto de los actores del mercado nacional y local, análisis de oportunidades en términos de nuevas fuentes de ingresos, cambios en las regulaciones del mercado, análisis estratégico del crecimiento del mercado, tamaño del mercado, crecimiento de las categorías de mercado, nichos de aplicación y dominio, aprobaciones y lanzamientos de productos, expansiones geográficas e innovaciones tecnológicas en el mercado. Para obtener más información sobre el mercado, contacte con Data Bridge Market Research para obtener un informe analítico. Nuestro equipo le ayudará a tomar decisiones informadas para impulsar el crecimiento del mercado.
Alcance del mercado de accidentes cerebrovasculares
El mercado se clasifica en cinco segmentos importantes según tipo, diagnóstico y tratamiento, género, usuario final y canal de distribución. El crecimiento de estos segmentos le ayudará a analizar los segmentos con menor crecimiento en las industrias y proporcionará a los usuarios una valiosa visión general del mercado y perspectivas que les ayudarán a tomar decisiones estratégicas para identificar las principales aplicaciones del mercado.
Tipo
- Accidente cerebrovascular isquémico
- Trombótica (trombosis cerebral)
- Embólico (embolia cerebral)
- Accidente cerebrovascular hemorrágico
- Hemorragia subaracnoidea
- Hemorragia intracerebral
- Accidente isquémico transitorio (AIT)
Diagnóstico y tratamiento
- Tratamiento
- Medicamento
- Por clase
- Medicamentos para la presión arterial
- Inhibidores de la enzima convertidora de angiotensina (ECA)
- Ramipril
- Lisinopril
- Enalapril
- Perindopril
- Otro
- Diuréticos tiazídicos
- Indapamida
- Bendroflumetiazida
- Espironolactona
- Amilorida
- Otro
- Bloqueadores de los canales de calcio
- Amlodipino
- Nifedipina
- Verapamilo
- Nicardipina
- Felodipina
- Nimodipina
- Otro
- Betabloqueantes
- Atenolol
- Bisoprolol
- Labetolol
- Otros
- Alfabloqueantes
- Doxazosina
- Otros
- Otros
- Inhibidores de la enzima convertidora de angiotensina (ECA)
- Medicamentos antiplaquetarios
- Aspirina
- Clopidogrel
- Dipiridamol
- Ticlopidina
- Otros
- Anticoagulantes
- Warfarina
- Apixabán
- Dabigatrán
- Heparina
- Rivaroxabán
- Otro
- Activador del plasminógeno tisular (TPA)
- Alteplasa
- Tenecteplasa
- Reteplasa
- Anistreplasa
- Otro
- Estatinas
- Atorvastatina
- Simvastatina
- Lovastatina
- Rosuvastatina
- Fluvastatina
- Pravastatina
- Pitavastatina
- Otros
- Vitamina K
- Medicación de apoyo
- Suplementos nutricionales
- Antipiréticos
- Otros
- Medicamentos para la presión arterial
- Por tipo de fármaco
- De marca
- Activase
- Edobaxan
- Coumadin
- Heparina Leo
- Duoplavina
- Aggrenox
- Retavase
- Jantoven
- Cathflo
- Otro
- De marca
- Genérico
- Por vía de administración
- Oral
- Tableta
- Cápsulas
- Otros
- Parenteral
- Intravenoso
- Subcutáneo
- Otros
- Oral
- Por modo de compra
- Prescripción
- Medicamentos de venta libre (OTC)
- Por tipo de terapia
- Terapia combinada
- Monoterapia
- Por vía de administración
- Cirugía
- Espirales embólicas
- Catéteres de aspiración
- recuperador de stents
- Clipaje quirúrgico
- Otros
- Otros Terapia
- Fisioterapia
- Terapia ocupacional
- Terapia del habla
- Otros
- Por clase
- Diagnóstico
- Prueba de imagen
- Tomografía computarizada (TC)
- Imágenes por resonancia magnética (IRM)
- Ecografía carotídea
- Angiografía cerebral
- Análisis de sangre
- Ecocardiograma
- Punción lumbar
- Otros
- Prueba de imagen
- Medicamento
Género
- Femenino
- Masculino
Usuario final
- Hospitales y clínicas
- Clínicas especializadas
- Centro de Cirugía Ambulatoria
- Cuidado domiciliario
- Laboratorios
- Otros
Canal de distribución
- Directo
- Minorista
- En línea
Análisis regional del mercado de accidentes cerebrovasculares
Se analiza el mercado y se proporcionan información y tendencias del tamaño del mercado por tipo, diagnóstico y tratamiento, género, usuario final y canal de distribución como se menciona anteriormente.
Los países cubiertos en el mercado son EE. UU., Canadá, México, Alemania, Francia, Reino Unido, Países Bajos, Suiza, Rusia, Italia, España, Turquía, Austria, Polonia, Noruega, Irlanda, Resto de Europa, China, Japón, India, Corea del Sur, Singapur, Malasia, Australia, Tailandia, Indonesia, Filipinas, Vietnam, Taiwán, Resto de Asia-Pacífico, Arabia Saudita, Emiratos Árabes Unidos, Sudáfrica, Egipto, Israel, Kuwait, Resto de Medio Oriente y África, Brasil, Argentina, Chile, Perú, Resto de Sudamérica.
Se espera que América del Norte domine el mercado mundial de accidentes cerebrovasculares debido a sus elevados gastos de atención médica, su infraestructura médica avanzada y sus programas integrales de atención de accidentes cerebrovasculares.
Asia-Pacífico es la región de más rápido crecimiento en el mercado mundial de accidentes cerebrovasculares en el período de pronóstico debido a la creciente conciencia de la prevención de accidentes cerebrovasculares, el aumento del acceso a la atención médica y una creciente población de ancianos.
La sección de países del informe también presenta los factores que impactan en cada mercado y los cambios en la regulación nacional que impactan las tendencias actuales y futuras del mercado. Datos como el análisis de la cadena de valor aguas abajo y aguas arriba, las tendencias técnicas, el análisis de las cinco fuerzas de Porter y los estudios de caso son algunos de los indicadores utilizados para pronosticar el escenario del mercado en cada país. Asimismo, se consideran la presencia y disponibilidad de marcas globales y los desafíos que enfrentan debido a la alta o escasa competencia de marcas locales y nacionales, el impacto de los aranceles nacionales y las rutas comerciales, al proporcionar un análisis de pronóstico de los datos nacionales.
Cuota de mercado de accidentes cerebrovasculares
El panorama competitivo del mercado ofrece detalles por competidor. Se incluye información general de la empresa, sus estados financieros, ingresos generados, potencial de mercado, inversión en investigación y desarrollo, nuevas iniciativas de mercado, presencia global, plantas de producción, capacidad de producción, fortalezas y debilidades de la empresa, lanzamiento de productos, alcance y variedad de productos, y dominio de las aplicaciones. Los datos anteriores se refieren únicamente al enfoque de mercado de las empresas.
Los líderes del mercado de accidentes cerebrovasculares que operan en el mercado son:
- Bristol-Myers Squibb Company (EE. UU.)
- Boehringer Ingelheim International GmbH (Alemania)
- F. Hoffmann-La Roche Ltd (Suiza)
- DAIICHI SANKYO COMPANY, LIMITED (Japón)
- Sanofi (Francia)
- Johnson & Johnson Services, Inc. (EE. UU.)
- Bayer AG (Alemania)
- Sandoz AG (Suiza)
- Pfizer Inc. (EE. UU.)
- Medtronic (Irlanda)
- Abbott (EE. UU.)
- Viatris Inc. (EE. UU.)
- AstraZeneca (Reino Unido)
- Penumbra, Inc. (EE. UU.)
- GLENMARK PHARMACEUTICALS LTD (India)
- Fresenius SE & Co. KGaA (Alemania)
- Teva Pharmaceuticals USA, Inc. (Israel)
- Lupino (India)
- Amneal Pharmaceuticals LLC (EE. UU.)
Últimos avances en el mercado de accidentes cerebrovasculares
- En julio de 2023, Roche anunció una nueva alianza con Alnylam para desarrollar y comercializar zilebesir, una terapia de ARNi en investigación, actualmente en fase 2, para el tratamiento de la hipertensión arterial. Esta colaboración combina la experiencia demostrada de Alnylam en terapia de ARNi con el alcance comercial global de Roche, su compromiso con la innovación y su deseo de transformar el panorama para los pacientes con enfermedades cardiovasculares graves.
- En septiembre de 2020, Daiichi Sankyo Company Limited anunció la presentación de una solicitud complementaria en Japón para la extensión de la aprobación del anticoagulante edoxabán (hidrato de benzoato de edoxabán) en pacientes de edad avanzada con insuficiencia no valvular y hemorragia grave. Riesgo. Esta solicitud se basa en los resultados de un ensayo clínico japonés de fase 3 (ensayo ELDERCARE-AF) en 984 pacientes con fibrilación auricular no valvular, mayores de 80 años, con alto riesgo de hemorragia y que no son aptos para otras terapias anticoagulantes disponibles. Daiichi Sankyo planea contribuir al tratamiento de pacientes de edad avanzada con fibrilación auricular no valvular ofreciendo una nueva opción terapéutica.
- En julio de 2022, Sandoz, el principal fabricante mundial de genéricos y biosimilares, anunció una inversión de aproximadamente 90 millones de dólares en sus instalaciones de Liubliana (Eslovenia) para establecer su Centro de Desarrollo Biofarmacéutico de Sandoz en 2026. Con esta inversión, la sede de Liubliana se convertirá en uno de los centros de desarrollo de biosimilares más importantes de Sandoz. La nueva oficina generará aproximadamente 200 nuevos empleos a tiempo completo y fortalecerá aún más la capacidad de la compañía en el desarrollo de biosimilares y productos farmacéuticos.
- En enero de 2023, Penumbra, Inc., compañía global de atención médica especializada en terapias innovadoras, anunció la aprobación y el lanzamiento de Lightning Flash™ por parte de la Administración de Alimentos y Medicamentos de los Estados Unidos (FDA), el sistema de trombectomía mecánica más avanzado y potente del mercado. Lightning Flash incorpora la nueva tecnología Lightning Intelligent Aspiration de Penumbra, que ahora cuenta con dos algoritmos de detección de coágulos. Combinada con una innovadora tecnología de catéter, Lightning Flash está diseñada para eliminar rápidamente grandes coágulos sanguíneos del cuerpo, incluyendo embolias venosas y embolias pulmonares (EP). Este lanzamiento ayudará a la compañía a ampliar su cartera de productos gracias a la excepcional trazabilidad de los resultados avanzados de esta nueva tecnología y a su capacidad única para diferenciar la sangre que fluye de los coágulos.
- En agosto de 2023, Lupin anunció el lanzamiento de Jeet, un programa de apoyo a pacientes dedicado a la salud cardiovascular. El lanzamiento de la iniciativa coincide con el 77.º Día de la Independencia de la India, que simboliza la liberación del estrés relacionado con las enfermedades y el camino hacia una vida más feliz y saludable. Jeet se convierte en un aliado de confianza en la atención cardiovascular al ofrecer diversos beneficios, como ahorros, asistencia médica, recordatorios de medicamentos y apoyo para un estilo de vida saludable. Jeet ofrece un enfoque holístico para mejorar la experiencia del médico y del paciente, fomentando la concienciación sobre las enfermedades cardiovasculares y sus comorbilidades. La aplicación incluye funciones diseñadas para fomentar un estilo de vida más saludable y promover la salud cardiovascular.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 LIMITATIONS
1.4 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
5 GLOBAL STROKE MARKET, REGULATORY FRAMEWORK
5.1 REGULATION IN U.S.
5.2 REGULATION IN EUROPE
5.3 REGULATION IN CHINA
5.4 REGULATION IN JAPAN
5.5 REGULATION IN SOUTH AFRICA
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING INCIDENCES OF STROKE DRIVE DEMAND FOR TREATMENTS
6.1.2 INCREASING NUMBER OF PATIENTS WITH HYPERTENSION AND CORONARY HEART DISEASES
6.1.3 INCREASING DIABETIC AND OBESE POPULATIONS ELEVATE STROKE RISKS
6.1.4 ADVANCEMENTS IN MEDICAL TECHNOLOGY IMPROVE STROKE CARE OUTCO.MES
6.2 RESTRAINTS
6.2.1 HIGH COST OF DIAGNOSIS
6.2.2 INCREASE IN PRODUCT RECALL
6.3 OPPORTUNITIES
6.3.1 DEVELOPMENT OF ADVANCED THERAPEUTICS FOR STROKES
6.3.2 EXPANSION IN STROKE REHABILITATION SERVICES
6.3.3 INNOVATIVE TREATMENTS IN PIPELINE FOR STROKE TREATMENT
6.4 CHALLENGES
6.4.1 FALSE DIAGNOSIS IN STROKES
6.4.2 COMPLICATIONS ASSOCIATED WITH MANAGING STROKE
7 GLOBAL STROKE MARKET, BY TYPE
7.1 OVERVIEW
7.2 ISCHEMIC STROKE
7.2.1 THROMBOTIC (CEREBRAL THROMBOSIS)
7.2.2 EMBOLIC (CEREBRAL EMBOLISM)
7.3 HEMORRHAGIC STROKE
7.3.1 SUBARACHNOID HEMORRHAGE
7.3.2 INTRACEREBRAL HEMORRHAGE
7.4 TRANSIENT ISCHEMIC ATTACT (TIA)
8 GLOBAL STROKE MARKET, BY GENDER
8.1 OVERVIEW
8.2 FEMALE
8.3 MALE
9 GLOBAL STROKE MARKET, BY DIAGNOSIS AND TREATMENT
9.1 OVERVIEW
9.2 TREATMENT
9.2.1 BY TREATMENT TYPE
9.2.1.1 MEDICATION
9.2.1.1.1 MEDICATION, BY CLASS
9.2.1.1.1.1 BLOOD PRESSURE MEDICINES
9.2.1.1.1.1.1 ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS
9.2.1.1.1.1.2 RAMIPRIL
9.2.1.1.1.1.3 LISINOPRIL
9.2.1.1.1.1.4 ENALAPRIL
9.2.1.1.1.1.5 PERINDOPRIL
9.2.1.1.1.1.6 OTHER
9.2.1.1.1.2 THIAZIDE DIURETICS
9.2.1.1.1.2.1 INDAPAMIDE
9.2.1.1.1.2.2 BENDROFLUMETHIAZIDE
9.2.1.1.1.2.3 SPIRONOLACTONE
9.2.1.1.1.2.4 AMILORIDE
9.2.1.1.1.2.5 OTHER
9.2.1.1.1.3 CALCIUM CHANNEL BLOCKERS
9.2.1.1.1.3.1 AMLODIPINE
9.2.1.1.1.3.2 NIFEDIPINE
9.2.1.1.1.3.3 VERAPAMIL
9.2.1.1.1.3.4 NICARDIPINE
9.2.1.1.1.3.5 FELODIPINE
9.2.1.1.1.3.6 NIMODIPINE
9.2.1.1.1.3.7 OTHERS
9.2.1.1.1.4 BETA BLOCKERS
9.2.1.1.1.4.1 ATENOLOL
9.2.1.1.1.4.2 BISOPROLOL
9.2.1.1.1.4.3 LABETOLOL
9.2.1.1.1.4.4 OTHERS
9.2.1.1.1.5 ALPHA-BLOCKERS
9.2.1.1.1.5.1 DOXAZOSIN
9.2.1.1.1.5.2 OTHERS
9.2.1.1.1.6 OTHERS
9.2.1.1.1.7 ANTIPLATELET DRUGS
9.2.1.1.1.7.1 ASPIRIN
9.2.1.1.1.7.2 CLOPIDOGREL
9.2.1.1.1.7.3 DIPYRIDAMOLE
9.2.1.1.1.7.4 TICLOPIDINE
9.2.1.1.1.7.5 OTHERS
9.2.1.1.1.8 ANTICOAGULANTS
9.2.1.1.1.8.1 WARFARIN
9.2.1.1.1.8.2 APIXABAN
9.2.1.1.1.8.3 DABIGATRAN
9.2.1.1.1.8.4 HEPARIN
9.2.1.1.1.8.5 RIVAROXABAN
9.2.1.1.1.8.6 OTHERS
9.2.1.1.1.9 TISSUE PLASMINOGEN ACTIVATOR (TPA)
9.2.1.1.1.9.1 ALTEPLASE
9.2.1.1.1.9.2 TENECTEPLASE
9.2.1.1.1.9.3 RETEPLASE
9.2.1.1.1.9.4 ANISTREPLASE
9.2.1.1.1.9.5 OTHERS
9.2.1.1.1.10 STATINS
9.2.1.1.1.10.1.1 ATORVASTATIN
9.2.1.1.1.10.1.2 SIMVASTATIN
9.2.1.1.1.10.1.3 LOVASTATIN
9.2.1.1.1.10.1.4 ROSUVASTATIN
9.2.1.1.1.10.1.5 FLUVASTATIN
9.2.1.1.1.10.1.6 PRAVASTATIN
9.2.1.1.1.10.1.7 PITAVASTATIN
9.2.1.1.1.10.1.8 OTHERS
9.2.1.1.1.11 VITAMIN K
9.2.1.1.1.12 SUPPORTIVE MEDICATION
9.2.1.1.1.12.1 NUTRITIONAL SUPPLEMENTS
9.2.1.1.1.12.2 ANTIPYRETICS
9.2.1.1.1.12.3 OTHERS
9.2.1.1.2 MEDICATION, BY DRUG TYPE
9.2.1.1.2.1 BRANDED
9.2.1.1.2.1.1 ACTIVASE
9.2.1.1.2.1.2 EDOBAXAN
9.2.1.1.2.1.3 COUMADIN
9.2.1.1.2.1.4 HEPARIN LEO
9.2.1.1.2.1.5 DUOPLAVIN
9.2.1.1.2.1.6 AGGRENOX
9.2.1.1.2.1.7 RETAVASE
9.2.1.1.2.1.8 JANTOVEN
9.2.1.1.2.1.9 CATHFLO
9.2.1.1.2.1.10 OTHER
9.2.1.1.2.2 GENERIC
9.2.1.1.3 MEDICATION, BY ROUTE OF ADMINISTRATION
9.2.1.1.3.1 ORAL
9.2.1.1.3.1.1 TABLET
9.2.1.1.3.1.2 CAPSULES
9.2.1.1.3.1.3 OTHERS
9.2.1.1.3.2 PARENTERAL
9.2.1.1.3.2.1 INTRAVENOUS
9.2.1.1.3.2.2 SUBCUTANEOUS
9.2.1.1.3.3 OTHERS
9.2.1.1.4 MEDICATION, BY MODE OF PURCHASE
9.2.1.1.4.1 PRESCRIPTION
9.2.1.1.4.2 OVER THE COUNTER (OTC)
9.2.1.1.5 MEDICATION, BY THERAPY TYPE
9.2.1.1.5.1 COMBINATION THERAPY
9.2.1.1.5.2 MONOTHERAPY
9.2.1.2 SURGERY
9.2.1.2.1 EMBOLIC COILS
9.2.1.2.2 ASPIRATION CATHETERS
9.2.1.2.3 STENT RETRIEVER
9.2.1.2.4 SURGICAL CLIPPING
9.2.1.2.5 OTHERS
9.2.1.3 OTHERS THERAPY
9.2.1.3.1 PHYSICAL THERAPY
9.2.1.3.2 OCCUPATIONAL THERAPY
9.2.1.3.3 SPEECH THERAPY
9.2.1.3.4 OTHERS
9.3 DIAGNOSIS
9.3.1 IMAGING TEST
9.3.1.1 COMPUTERIZED TOMOGRAPHY (CT) SCAN
9.3.1.2 MAGNETIC RESONANCE IMAGING (MRI)
9.3.1.3 CAROTID ULTRASOUND
9.3.1.4 CEREBRAL ANGIOGRAM
9.3.2 BLOOD TEST
9.3.3 ECHOCARDIOGRAM
9.3.4 LUMBAR PUNCTURE
9.3.5 OTHERS
10 GLOBAL STROKE MARKET, BY DISTRIBUTION CHANNEL
10.1 OVERVIEW
10.2 DIRECT
10.3 RETAIL
10.4 ONLINE
11 GLOBAL STROKE MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS & CLINICS
11.3 SPECIALTY CLINICS
11.4 AMBULATORY SURGICAL CENTER
11.5 HOMECARE
11.6 LABORATORIES
11.7 OTHERS
12 GLOBAL STROKE MARKET, BY REGION
12.1 OVERVIEW
12.2 NORTH AMERICA
12.2.1 U.S
12.2.2 CANADA
12.2.3 MEXICO
12.3 EUROPE
12.3.1 GERMANY
12.3.2 U.K.
12.3.3 FRANCE
12.3.4 ITALY
12.3.5 SPAIN
12.3.6 RUSSIA
12.3.7 NETHERLANDS
12.3.8 SWITZERLAND
12.3.9 TURKEY
12.3.10 AUSTRIA
12.3.11 POLAND
12.3.12 NORWAY
12.3.13 IRELAND
12.3.14 REST OF EUROPE
12.4 ASIA-PACIFIC
12.4.1 CHINA
12.4.2 JAPAN
12.4.3 INDIA
12.4.4 AUSTRALIA
12.4.5 SOUTH KOREA
12.4.6 SINGAPORE
12.4.7 THAILAND
12.4.8 PHILIPPINES
12.4.9 MALAYSIA
12.4.10 INDONESIA
12.4.11 VIETNAM
12.4.12 TAIWAN
12.4.13 REST OF ASIA-PACIFIC
12.5 SOUTH AMERICA
12.5.1 BRAZIL
12.5.2 ARGENTINA
12.5.3 CHILE
12.5.4 PERU
12.5.5 REST OF SOUTH AMERICA
12.6 MIDDLE EAST AND AFRICA
12.6.1 SOUTH AFRICA
12.6.2 SAUDI ARABIA
12.6.3 EGYPT
12.6.4 U.A.E.
12.6.5 ISRAEL
12.6.6 KUWAIT
13 GLOBAL STROKE MARKET, COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: GLOBAL
13.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
13.3 COMPANY SHARE ANALYSIS: EUROPE
13.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
14 SWOT ANALYSIS
15 COMPANY PROFILES
15.1 BRISTOL-MYERS SQUIBB COMPANY
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.2 F. HOFFMANN-LA ROCHE LTD
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT
15.3 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
15.3.1 COMPANY SNAPSHOT
15.3.2 COMPANY SHARE ANALYSIS
15.3.3 PRODUCT PORTFOLIO
15.3.4 RECENT DEVELOPMENT
15.4 DAIICHI SANKYO COMPANY, LIMITED
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENT
15.5 SANOFI
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.6 ABBOTT
15.6.1 COMPANY SNAPSHOT
15.6.2 REVENUE ANALYSIS
15.6.3 PRODUCT PORTFOLIO
15.6.4 RECENT DEVELOPMENT
15.7 AMNEAL PHARMACEUTICALS LLC
15.7.1 COMPANY SNAPSHOT
15.7.2 REVENUE ANALYSIS
15.7.3 PRODUCT PORTFOLIO
15.7.4 RECENT DEVELOPMENT
15.8 ASTRAZENECA
15.8.1 COMPANY SNAPSHOT
15.8.2 REVENUE ANALYSIS
15.8.3 PRODUCT PORTFOLIO
15.8.4 RECENT DEVELOPMENT
15.9 BAYER AG
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENT
15.1 FRESENIUS SE & CO. KGAA
15.10.1 COMPANY SNAPSHOT
15.10.2 REVENUE ANALYSIS
15.10.3 PRODUCT PORTFOLIO
15.10.4 RECENT DEVELOPMENT
15.11 GLENMARK PHARMACEUTICALS LTD.
15.11.1 COMPANY SNAPSHOT
15.11.2 REVENUE ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENT
15.12 JOHNSON & JOHNSON SERVICES, INC.
15.12.1 COMPANY SNAPSHOT
15.12.2 REVENUE ANALYSIS
15.12.3 PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENT
15.13 LUPIN
15.13.1 COMPANY SNAPSHOT
15.13.2 REVENUE ANALYSIS
15.13.3 PRODUCT PORTFOLIO
15.13.4 RECENT DEVELOPMENT
15.14 MEDTRONIC
15.14.1 COMPANY SNAPSHOT
15.14.2 REVENUE ANALYSIS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENT
15.15 PENUMBRA, INC.
15.15.1 COMPANY SNAPSHOT
15.15.2 REVENUE ANALYSIS
15.15.3 PRODUCT PORTFOLIO
15.15.4 RECENT DEVELOPMENT
15.16 PFIZER INC.
15.16.1 COMPANY SNAPSHOT
15.16.2 REVENUE ANALYSIS
15.16.3 PRODUCT PORTFOLIO
15.16.4 RECENT DEVELOPMENT
15.17 SANDOZ AG
15.17.1 COMPANY SNAPSHOT
15.17.2 REVENUE ANALYSIS
15.17.3 PRODUCT PORTFOLIO
15.17.4 RECENT DEVELOPMENT
15.18 TEVA PHARMACEUTICALS USA, INC.
15.18.1 COMPANY SNAPSHOT
15.18.2 REVENUE ANALYSIS
15.18.3 PRODUCT PORTFOLIO
15.18.4 RECENT DEVELOPMENT
15.19 VIATRIS INC.
15.19.1 COMPANY SNAPSHOT
15.19.2 REVENUE ANALYSIS
15.19.3 PRODUCT PORTFOLIO
15.19.4 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
Lista de Tablas
TABLE 1 GLOBAL STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 2 GLOBAL ISCHEMIC STROKE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 3 GLOBAL ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 4 GLOBAL HEMORRHAGIC STROKE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 5 GLOBAL HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 6 GLOBAL TRANSIENT ISCHEMIC ATTACT (TIA) IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 7 GLOBAL STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 8 GLOBAL FEMALE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 9 GLOBAL MALE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 10 GLOBAL STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 11 GLOBAL TREATMENT IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 12 GLOBAL TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 13 GLOBAL MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 14 GLOBAL BLOOD PRESSURE MEDICINES IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 15 GLOBAL ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 16 GLOBAL THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 17 GLOBAL CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 18 GLOBAL BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 19 GLOBAL ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 20 GLOBAL ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 21 GLOBAL ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 22 GLOBAL TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 23 GLOBAL STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 24 GLOBAL SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 25 GLOBAL MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 26 GLOBAL BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 27 GLOBAL MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 28 GLOBAL ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 29 GLOBAL PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 30 GLOBAL MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 31 GLOBAL MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 32 GLOBAL SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 33 GLOBAL OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 34 GLOBAL DIAGNOSIS IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 35 GLOBAL DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 36 GLOBAL IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 37 GLOBAL STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 38 GLOBAL DIRECT IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 39 GLOBAL RETAIL IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 40 GLOBAL ONLINE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 41 GLOBAL STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 42 GLOBAL HOSPITALS & CLINICS IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 43 GLOBAL SPECIALTY CLINICS IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 44 GLOBAL AMBULATORY SURGICAL CENTER IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 45 GLOBAL HOMECARE IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 46 GLOBAL LABORATORIES IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 47 GLOBAL OTHERS IN STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 48 GLOBAL STROKE MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 49 NORTH AMERICA STROKE MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 50 NORTH-AMERICA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 51 NORTH-AMERICA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 52 NORTH-AMERICA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 53 NORTH-AMERICA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 54 NORTH-AMERICA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 55 NORTH-AMERICA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 56 NORTH-AMERICA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 57 NORTH-AMERICA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 58 NORTH-AMERICA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 59 NORTH-AMERICA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 60 NORTH-AMERICA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 61 NORTH-AMERICA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 62 NORTH-AMERICA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 63 NORTH-AMERICA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 64 NORTH-AMERICA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 65 NORTH-AMERICA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 66 NORTH-AMERICA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 67 NORTH-AMERICA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 68 NORTH-AMERICA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 69 NORTH-AMERICA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 70 NORTH-AMERICA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 71 NORTH-AMERICA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 72 NORTH-AMERICA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 73 NORTH-AMERICA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 74 NORTH-AMERICA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 75 NORTH-AMERICA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 76 NORTH-AMERICA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 77 NORTH-AMERICA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 78 NORTH-AMERICA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 79 NORTH-AMERICA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 80 NORTH-AMERICA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 81 U.S. STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 82 U.S. ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 83 U.S. HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 84 U.S. STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 85 U.S. TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 86 U.S. MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 87 U.S. BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 88 U.S. ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 89 U.S. THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 90 U.S. CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 91 U.S. BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 92 U.S. ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 93 U.S. ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 94 U.S. ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 95 U.S. TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 96 U.S. STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 97 U.S. SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 98 U.S. MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 99 U.S. BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 100 U.S. MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 101 U.S. ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 102 U.S. PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 103 U.S. MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 104 U.S. MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 105 U.S. SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 106 U.S. OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 107 U.S. DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 108 U.S. IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 109 U.S. STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 110 U.S. STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 111 U.S. STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 112 CANADA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 113 CANADA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 114 CANADA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 115 CANADA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 116 CANADA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 117 CANADA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 118 CANADA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 119 CANADA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 120 CANADA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 121 CANADA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 122 CANADA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 123 CANADA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 124 CANADA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 125 CANADA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 126 CANADA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 127 CANADA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 128 CANADA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 129 CANADA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 130 CANADA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 131 CANADA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 132 CANADA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 133 CANADA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 134 CANADA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 135 CANADA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 136 CANADA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 137 CANADA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 138 CANADA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 139 CANADA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 140 CANADA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 141 CANADA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 142 CANADA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 143 MEXICO STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 144 MEXICO ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 145 MEXICO HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 146 MEXICO STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 147 MEXICO TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 148 MEXICO MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 149 MEXICO BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 150 MEXICO ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 151 MEXICO THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 152 MEXICO CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 153 MEXICO BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 154 MEXICO ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 155 MEXICO ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 156 MEXICO ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 157 MEXICO TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 158 MEXICO STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 159 MEXICO SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 160 MEXICO MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 161 MEXICO BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 162 MEXICO MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 163 MEXICO ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 164 MEXICO PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 165 MEXICO MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 166 MEXICO MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 167 MEXICO SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 168 MEXICO OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 169 MEXICO DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 170 MEXICO IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 171 MEXICO STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 172 MEXICO STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 173 MEXICO STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 174 EUROPE STROKE MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 175 EUROPE STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 176 EUROPE ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 177 EUROPE HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 178 EUROPE STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 179 EUROPE TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 180 EUROPE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 181 EUROPE BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 182 EUROPE ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 183 EUROPE THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 184 EUROPE CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 185 EUROPE BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 186 EUROPE ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 187 EUROPE ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 188 EUROPE ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 189 EUROPE TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 190 EUROPE STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 191 EUROPE SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 192 EUROPE MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 193 EUROPE BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 194 EUROPE MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 195 EUROPE ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 196 EUROPE PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 197 EUROPE MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 198 EUROPE MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 199 EUROPE SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 200 EUROPE OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 201 EUROPE DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 202 EUROPE IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 203 EUROPE STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 204 EUROPE STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 205 EUROPE STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 206 GERMANY STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 207 GERMANY ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 208 GERMANY HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 209 GERMANY STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 210 GERMANY TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 211 GERMANY MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 212 GERMANY BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 213 GERMANY ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 214 GERMANY THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 215 GERMANY CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 216 GERMANY BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 217 GERMANY ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 218 GERMANY ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 219 GERMANY ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 220 GERMANY TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 221 GERMANY STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 222 GERMANY SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 223 GERMANY MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 224 GERMANY BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 225 GERMANY MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 226 GERMANY ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 227 GERMANY PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 228 GERMANY MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 229 GERMANY MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 230 GERMANY SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 231 GERMANY OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 232 GERMANY DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 233 GERMANY IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 234 GERMANY STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 235 GERMANY STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 236 GERMANY STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 237 U.K. STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 238 U.K. ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 239 U.K. HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 240 U.K. STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 241 U.K. TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 242 U.K. MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 243 U.K. BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 244 U.K. ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 245 U.K. THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 246 U.K. CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 247 U.K. BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 248 U.K. ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 249 U.K. ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 250 U.K. ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 251 U.K. TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 252 U.K. STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 253 U.K. SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 254 U.K. MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 255 U.K. BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 256 U.K. MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 257 U.K. ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 258 U.K. PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 259 U.K. MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 260 U.K. MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 261 U.K. SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 262 U.K. OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 263 U.K. DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 264 U.K. IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 265 U.K. STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 266 U.K. STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 267 U.K. STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 268 FRANCE STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 269 FRANCE ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 270 FRANCE HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 271 FRANCE STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 272 FRANCE TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 273 FRANCE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 274 FRANCE BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 275 FRANCE ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 276 FRANCE THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 277 FRANCE CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 278 FRANCE BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 279 FRANCE ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 280 FRANCE ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 281 FRANCE ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 282 FRANCE TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 283 FRANCE STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 284 FRANCE SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 285 FRANCE MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 286 FRANCE BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 287 FRANCE MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 288 FRANCE ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 289 FRANCE PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 290 FRANCE MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 291 FRANCE MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 292 FRANCE SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 293 FRANCE OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 294 FRANCE DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 295 FRANCE IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 296 FRANCE STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 297 FRANCE STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 298 FRANCE STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 299 ITALY STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 300 ITALY ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 301 ITALY HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 302 ITALY STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 303 ITALY TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 304 ITALY MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 305 ITALY BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 306 ITALY ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 307 ITALY THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 308 ITALY CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 309 ITALY BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 310 ITALY ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 311 ITALY ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 312 ITALY ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 313 ITALY TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 314 ITALY STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 315 ITALY SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 316 ITALY MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 317 ITALY BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 318 ITALY MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 319 ITALY ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 320 ITALY PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 321 ITALY MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 322 ITALY MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 323 ITALY SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 324 ITALY OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 325 ITALY DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 326 ITALY IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 327 ITALY STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 328 ITALY STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 329 ITALY STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 330 SPAIN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 331 SPAIN ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 332 SPAIN HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 333 SPAIN STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 334 SPAIN TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 335 SPAIN MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 336 SPAIN BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 337 SPAIN ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 338 SPAIN THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 339 SPAIN CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 340 SPAIN BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 341 SPAIN ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 342 SPAIN ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 343 SPAIN ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 344 SPAIN TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 345 SPAIN STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 346 SPAIN SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 347 SPAIN MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 348 SPAIN BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 349 SPAIN MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 350 SPAIN ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 351 SPAIN PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 352 SPAIN MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 353 SPAIN MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 354 SPAIN SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 355 SPAIN OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 356 SPAIN DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 357 SPAIN IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 358 SPAIN STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 359 SPAIN STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 360 SPAIN STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 361 RUSSIA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 362 RUSSIA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 363 RUSSIA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 364 RUSSIA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 365 RUSSIA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 366 RUSSIA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 367 RUSSIA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 368 RUSSIA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 369 RUSSIA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 370 RUSSIA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 371 RUSSIA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 372 RUSSIA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 373 RUSSIA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 374 RUSSIA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 375 RUSSIA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 376 RUSSIA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 377 RUSSIA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 378 RUSSIA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 379 RUSSIA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 380 RUSSIA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 381 RUSSIA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 382 RUSSIA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 383 RUSSIA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 384 RUSSIA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 385 RUSSIA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 386 RUSSIA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 387 RUSSIA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 388 RUSSIA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 389 RUSSIA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 390 RUSSIA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 391 RUSSIA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 392 NETHERLANDS STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 393 NETHERLANDS ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 394 NETHERLANDS HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 395 NETHERLANDS STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 396 NETHERLANDS TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 397 NETHERLANDS MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 398 NETHERLANDS BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 399 NETHERLANDS ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 400 NETHERLANDS THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 401 NETHERLANDS CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 402 NETHERLANDS BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 403 NETHERLANDS ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 404 NETHERLANDS ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 405 NETHERLANDS ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 406 NETHERLANDS TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 407 NETHERLANDS STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 408 NETHERLANDS SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 409 NETHERLANDS MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 410 NETHERLANDS BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 411 NETHERLANDS MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 412 NETHERLANDS ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 413 NETHERLANDS PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 414 NETHERLANDS MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 415 NETHERLANDS MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 416 NETHERLANDS SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 417 NETHERLANDS OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 418 NETHERLANDS DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 419 NETHERLANDS IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 420 NETHERLANDS STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 421 NETHERLANDS STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 422 NETHERLANDS STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 423 SWITZERLAND STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 424 SWITZERLAND ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 425 SWITZERLAND HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 426 SWITZERLAND STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 427 SWITZERLAND TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 428 SWITZERLAND MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 429 SWITZERLAND BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 430 SWITZERLAND ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 431 SWITZERLAND THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 432 SWITZERLAND CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 433 SWITZERLAND BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 434 SWITZERLAND ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 435 SWITZERLAND ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 436 SWITZERLAND ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 437 SWITZERLAND TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 438 SWITZERLAND STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 439 SWITZERLAND SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 440 SWITZERLAND MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 441 SWITZERLAND BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 442 SWITZERLAND MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 443 SWITZERLAND ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 444 SWITZERLAND PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 445 SWITZERLAND MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 446 SWITZERLAND MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 447 SWITZERLAND SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 448 SWITZERLAND OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 449 SWITZERLAND DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 450 SWITZERLAND IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 451 SWITZERLAND STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 452 SWITZERLAND STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 453 SWITZERLAND STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 454 TURKEY STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 455 TURKEY ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 456 TURKEY HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 457 TURKEY STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 458 TURKEY TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 459 TURKEY MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 460 TURKEY BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 461 TURKEY ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 462 TURKEY THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 463 TURKEY CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 464 TURKEY BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 465 TURKEY ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 466 TURKEY ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 467 TURKEY ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 468 TURKEY TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 469 TURKEY STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 470 TURKEY SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 471 TURKEY MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 472 TURKEY BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 473 TURKEY MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 474 TURKEY ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 475 TURKEY PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 476 TURKEY MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 477 TURKEY MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 478 TURKEY SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 479 TURKEY OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 480 TURKEY DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 481 TURKEY IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 482 TURKEY STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 483 TURKEY STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 484 TURKEY STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 485 AUSTRIA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 486 AUSTRIA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 487 AUSTRIA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 488 AUSTRIA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 489 AUSTRIA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 490 AUSTRIA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 491 AUSTRIA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 492 AUSTRIA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 493 AUSTRIA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 494 AUSTRIA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 495 AUSTRIA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 496 AUSTRIA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 497 AUSTRIA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 498 AUSTRIA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 499 AUSTRIA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 500 AUSTRIA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 501 AUSTRIA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 502 AUSTRIA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 503 AUSTRIA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 504 AUSTRIA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 505 AUSTRIA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 506 AUSTRIA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 507 AUSTRIA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 508 AUSTRIA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 509 AUSTRIA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 510 AUSTRIA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 511 AUSTRIA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 512 AUSTRIA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 513 AUSTRIA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 514 AUSTRIA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 515 AUSTRIA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 516 POLAND STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 517 POLAND ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 518 POLAND HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 519 POLAND STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 520 POLAND TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 521 POLAND MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 522 POLAND BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 523 POLAND ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 524 POLAND THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 525 POLAND CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 526 POLAND BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 527 POLAND ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 528 POLAND ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 529 POLAND ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 530 POLAND TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 531 POLAND STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 532 POLAND SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 533 POLAND MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 534 POLAND BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 535 POLAND MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 536 POLAND ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 537 POLAND PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 538 POLAND MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 539 POLAND MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 540 POLAND SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 541 POLAND OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 542 POLAND DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 543 POLAND IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 544 POLAND STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 545 POLAND STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 546 POLAND STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 547 NORWAY STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 548 NORWAY ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 549 NORWAY HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 550 NORWAY STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 551 NORWAY TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 552 NORWAY MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 553 NORWAY BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 554 NORWAY ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 555 NORWAY THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 556 NORWAY CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 557 NORWAY BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 558 NORWAY ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 559 NORWAY ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 560 NORWAY ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 561 NORWAY TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 562 NORWAY STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 563 NORWAY SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 564 NORWAY MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 565 NORWAY BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 566 NORWAY MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 567 NORWAY ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 568 NORWAY PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 569 NORWAY MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 570 NORWAY MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 571 NORWAY SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 572 NORWAY OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 573 NORWAY DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 574 NORWAY IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 575 NORWAY STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 576 NORWAY STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 577 NORWAY STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 578 IRELAND STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 579 IRELAND ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 580 IRELAND HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 581 IRELAND STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 582 IRELAND TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 583 IRELAND MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 584 IRELAND BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 585 IRELAND ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 586 IRELAND THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 587 IRELAND CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 588 IRELAND BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 589 IRELAND ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 590 IRELAND ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 591 IRELAND ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 592 IRELAND TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 593 IRELAND STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 594 IRELAND SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 595 IRELAND MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 596 IRELAND BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 597 IRELAND MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 598 IRELAND ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 599 IRELAND PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 600 IRELAND MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 601 IRELAND MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 602 IRELAND SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 603 IRELAND OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 604 IRELAND DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 605 IRELAND IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 606 IRELAND STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 607 IRELAND STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 608 IRELAND STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 609 REST OF EUROPE STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 610 ASIA-PACIFIC STROKE MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 611 ASIA-PACIFIC STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 612 ASIA-PACIFIC ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 613 ASIA-PACIFIC HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 614 ASIA-PACIFIC STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 615 ASIA-PACIFIC TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 616 ASIA-PACIFIC MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 617 ASIA-PACIFIC BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 618 ASIA-PACIFIC ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 619 ASIA-PACIFIC THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 620 ASIA-PACIFIC CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 621 ASIA-PACIFIC BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 622 ASIA-PACIFIC ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 623 ASIA-PACIFIC ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 624 ASIA-PACIFIC ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 625 ASIA-PACIFIC TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 626 ASIA-PACIFIC STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 627 ASIA-PACIFIC SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 628 ASIA-PACIFIC MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 629 ASIA-PACIFIC BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 630 ASIA-PACIFIC MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 631 ASIA-PACIFIC ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 632 ASIA-PACIFIC PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 633 ASIA-PACIFIC MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 634 ASIA-PACIFIC MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 635 ASIA-PACIFIC SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 636 ASIA-PACIFIC OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 637 ASIA-PACIFIC DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 638 ASIA-PACIFIC IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 639 ASIA-PACIFIC STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 640 ASIA-PACIFIC STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 641 ASIA-PACIFIC STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 642 CHINA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 643 CHINA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 644 CHINA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 645 CHINA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 646 CHINA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 647 CHINA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 648 CHINA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 649 CHINA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 650 CHINA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 651 CHINA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 652 CHINA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 653 CHINA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 654 CHINA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 655 CHINA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 656 CHINA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 657 CHINA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 658 CHINA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 659 CHINA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 660 CHINA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 661 CHINA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 662 CHINA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 663 CHINA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 664 CHINA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 665 CHINA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 666 CHINA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 667 CHINA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 668 CHINA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 669 CHINA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 670 CHINA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 671 CHINA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 672 CHINA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 673 JAPAN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 674 JAPAN ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 675 JAPAN HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 676 JAPAN STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 677 JAPAN TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 678 JAPAN MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 679 JAPAN BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 680 JAPAN ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 681 JAPAN THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 682 JAPAN CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 683 JAPAN BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 684 JAPAN ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 685 JAPAN ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 686 JAPAN ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 687 JAPAN TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 688 JAPAN STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 689 JAPAN SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 690 JAPAN MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 691 JAPAN BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 692 JAPAN MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 693 JAPAN ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 694 JAPAN PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 695 JAPAN MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 696 JAPAN MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 697 JAPAN SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 698 JAPAN OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 699 JAPAN DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 700 JAPAN IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 701 JAPAN STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 702 JAPAN STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 703 JAPAN STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 704 INDIA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 705 INDIA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 706 INDIA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 707 INDIA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 708 INDIA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 709 INDIA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 710 INDIA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 711 INDIA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 712 INDIA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 713 INDIA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 714 INDIA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 715 INDIA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 716 INDIA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 717 INDIA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 718 INDIA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 719 INDIA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 720 INDIA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 721 INDIA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 722 INDIA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 723 INDIA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 724 INDIA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 725 INDIA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 726 INDIA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 727 INDIA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 728 INDIA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 729 INDIA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 730 INDIA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 731 INDIA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 732 INDIA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 733 INDIA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 734 INDIA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 735 AUSTRALIA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 736 AUSTRALIA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 737 AUSTRALIA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 738 AUSTRALIA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 739 AUSTRALIA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 740 AUSTRALIA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 741 AUSTRALIA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 742 AUSTRALIA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 743 AUSTRALIA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 744 AUSTRALIA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 745 AUSTRALIA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 746 AUSTRALIA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 747 AUSTRALIA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 748 AUSTRALIA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 749 AUSTRALIA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 750 AUSTRALIA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 751 AUSTRALIA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 752 AUSTRALIA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 753 AUSTRALIA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 754 AUSTRALIA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 755 AUSTRALIA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 756 AUSTRALIA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 757 AUSTRALIA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 758 AUSTRALIA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 759 AUSTRALIA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 760 AUSTRALIA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 761 AUSTRALIA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 762 AUSTRALIA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 763 AUSTRALIA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 764 AUSTRALIA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 765 AUSTRALIA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 766 SOUTH KOREA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 767 SOUTH KOREA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 768 SOUTH KOREA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 769 SOUTH KOREA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 770 SOUTH KOREA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 771 SOUTH KOREA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 772 SOUTH KOREA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 773 SOUTH KOREA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 774 SOUTH KOREA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 775 SOUTH KOREA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 776 SOUTH KOREA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 777 SOUTH KOREA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 778 SOUTH KOREA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 779 SOUTH KOREA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 780 SOUTH KOREA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 781 SOUTH KOREA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 782 SOUTH KOREA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 783 SOUTH KOREA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 784 SOUTH KOREA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 785 SOUTH KOREA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 786 SOUTH KOREA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 787 SOUTH KOREA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 788 SOUTH KOREA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 789 SOUTH KOREA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 790 SOUTH KOREA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 791 SOUTH KOREA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 792 SOUTH KOREA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 793 SOUTH KOREA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 794 SOUTH KOREA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 795 SOUTH KOREA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 796 SOUTH KOREA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 797 SINGAPORE STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 798 SINGAPORE ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 799 SINGAPORE HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 800 SINGAPORE STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 801 SINGAPORE TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 802 SINGAPORE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 803 SINGAPORE BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 804 SINGAPORE ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 805 SINGAPORE THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 806 SINGAPORE CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 807 SINGAPORE BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 808 SINGAPORE ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 809 SINGAPORE ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 810 SINGAPORE ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 811 SINGAPORE TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 812 SINGAPORE STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 813 SINGAPORE SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 814 SINGAPORE MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 815 SINGAPORE BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 816 SINGAPORE MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 817 SINGAPORE ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 818 SINGAPORE PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 819 SINGAPORE MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 820 SINGAPORE MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 821 SINGAPORE SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 822 SINGAPORE OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 823 SINGAPORE DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 824 SINGAPORE IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 825 SINGAPORE STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 826 SINGAPORE STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 827 SINGAPORE STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 828 THAILAND STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 829 THAILAND ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 830 THAILAND HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 831 THAILAND STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 832 THAILAND TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 833 THAILAND MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 834 THAILAND BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 835 THAILAND ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 836 THAILAND THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 837 THAILAND CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 838 THAILAND BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 839 THAILAND ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 840 THAILAND ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 841 THAILAND ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 842 THAILAND TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 843 THAILAND STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 844 THAILAND SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 845 THAILAND MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 846 THAILAND BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 847 THAILAND MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 848 THAILAND ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 849 THAILAND PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 850 THAILAND MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 851 THAILAND MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 852 THAILAND SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 853 THAILAND OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 854 THAILAND DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 855 THAILAND IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 856 THAILAND STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 857 THAILAND STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 858 THAILAND STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 859 PHILIPPINES STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 860 PHILIPPINES ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 861 PHILIPPINES HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 862 PHILIPPINES STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 863 PHILIPPINES TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 864 PHILIPPINES MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 865 PHILIPPINES BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 866 PHILIPPINES ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 867 PHILIPPINES THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 868 PHILIPPINES CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 869 PHILIPPINES BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 870 PHILIPPINES ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 871 PHILIPPINES ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 872 PHILIPPINES ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 873 PHILIPPINES TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 874 PHILIPPINES STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 875 PHILIPPINES SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 876 PHILIPPINES MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 877 PHILIPPINES BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 878 PHILIPPINES MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 879 PHILIPPINES ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 880 PHILIPPINES PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 881 PHILIPPINES MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 882 PHILIPPINES MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 883 PHILIPPINES SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 884 PHILIPPINES OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 885 PHILIPPINES DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 886 PHILIPPINES IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 887 PHILIPPINES STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 888 PHILIPPINES STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 889 PHILIPPINES STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 890 MALAYSIA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 891 MALAYSIA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 892 MALAYSIA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 893 MALAYSIA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 894 MALAYSIA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 895 MALAYSIA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 896 MALAYSIA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 897 MALAYSIA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 898 MALAYSIA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 899 MALAYSIA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 900 MALAYSIA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 901 MALAYSIA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 902 MALAYSIA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 903 MALAYSIA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 904 MALAYSIA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 905 MALAYSIA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 906 MALAYSIA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 907 MALAYSIA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 908 MALAYSIA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 909 MALAYSIA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 910 MALAYSIA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 911 MALAYSIA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 912 MALAYSIA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 913 MALAYSIA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 914 MALAYSIA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 915 MALAYSIA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 916 MALAYSIA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 917 MALAYSIA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 918 MALAYSIA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 919 MALAYSIA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 920 MALAYSIA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 921 INDONESIA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 922 INDONESIA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 923 INDONESIA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 924 INDONESIA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 925 INDONESIA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 926 INDONESIA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 927 INDONESIA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 928 INDONESIA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 929 INDONESIA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 930 INDONESIA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 931 INDONESIA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 932 INDONESIA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 933 INDONESIA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 934 INDONESIA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 935 INDONESIA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 936 INDONESIA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 937 INDONESIA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 938 INDONESIA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 939 INDONESIA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 940 INDONESIA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 941 INDONESIA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 942 INDONESIA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 943 INDONESIA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 944 INDONESIA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 945 INDONESIA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 946 INDONESIA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 947 INDONESIA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 948 INDONESIA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 949 INDONESIA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 950 INDONESIA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 951 INDONESIA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 952 VIETNAM STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 953 VIETNAM ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 954 VIETNAM HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 955 VIETNAM STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 956 VIETNAM TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 957 VIETNAM MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 958 VIETNAM BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 959 VIETNAM ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 960 VIETNAM THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 961 VIETNAM CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 962 VIETNAM BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 963 VIETNAM ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 964 VIETNAM ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 965 VIETNAM ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 966 VIETNAM TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 967 VIETNAM STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 968 VIETNAM SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 969 VIETNAM MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 970 VIETNAM BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 971 VIETNAM MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 972 VIETNAM ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 973 VIETNAM PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 974 VIETNAM MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 975 VIETNAM MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 976 VIETNAM SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 977 VIETNAM OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 978 VIETNAM DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 979 VIETNAM IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 980 VIETNAM STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 981 VIETNAM STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 982 VIETNAM STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 983 TAIWAN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 984 TAIWAN ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 985 TAIWAN HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 986 TAIWAN STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 987 TAIWAN TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 988 TAIWAN MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 989 TAIWAN BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 990 TAIWAN ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 991 TAIWAN THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 992 TAIWAN CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 993 TAIWAN BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 994 TAIWAN ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 995 TAIWAN ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 996 TAIWAN ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 997 TAIWAN TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 998 TAIWAN STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 999 TAIWAN SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1000 TAIWAN MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1001 TAIWAN BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1002 TAIWAN MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 1003 TAIWAN ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1004 TAIWAN PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1005 TAIWAN MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 1006 TAIWAN MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1007 TAIWAN SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1008 TAIWAN OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1009 TAIWAN DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 1010 TAIWAN IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1011 TAIWAN STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 1012 TAIWAN STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 1013 TAIWAN STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 1014 REST OF ASIA-PACIFIC STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1015 SOUTH AMERICA STROKE MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 1016 SOUTH AMERICA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1017 SOUTH AMERICA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1018 SOUTH AMERICA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1019 SOUTH AMERICA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 1020 SOUTH AMERICA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1021 SOUTH AMERICA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1022 SOUTH AMERICA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1023 SOUTH AMERICA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1024 SOUTH AMERICA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1025 SOUTH AMERICA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1026 SOUTH AMERICA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1027 SOUTH AMERICA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1028 SOUTH AMERICA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1029 SOUTH AMERICA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1030 SOUTH AMERICA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1031 SOUTH AMERICA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1032 SOUTH AMERICA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1033 SOUTH AMERICA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1034 SOUTH AMERICA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1035 SOUTH AMERICA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 1036 SOUTH AMERICA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1037 SOUTH AMERICA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1038 SOUTH AMERICA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 1039 SOUTH AMERICA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1040 SOUTH AMERICA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1041 SOUTH AMERICA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1042 SOUTH AMERICA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 1043 SOUTH AMERICA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1044 SOUTH AMERICA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 1045 SOUTH AMERICA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 1046 SOUTH AMERICA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 1047 BRAZIL STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1048 BRAZIL ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1049 BRAZIL HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1050 BRAZIL STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 1051 BRAZIL TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1052 BRAZIL MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1053 BRAZIL BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1054 BRAZIL ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1055 BRAZIL THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1056 BRAZIL CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1057 BRAZIL BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1058 BRAZIL ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1059 BRAZIL ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1060 BRAZIL ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1061 BRAZIL TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1062 BRAZIL STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1063 BRAZIL SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1064 BRAZIL MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1065 BRAZIL BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1066 BRAZIL MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 1067 BRAZIL ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1068 BRAZIL PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1069 BRAZIL MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 1070 BRAZIL MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1071 BRAZIL SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1072 BRAZIL OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1073 BRAZIL DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 1074 BRAZIL IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1075 BRAZIL STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 1076 BRAZIL STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 1077 BRAZIL STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 1078 ARGENTINA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1079 ARGENTINA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1080 ARGENTINA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1081 ARGENTINA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 1082 ARGENTINA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1083 ARGENTINA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1084 ARGENTINA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1085 ARGENTINA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1086 ARGENTINA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1087 ARGENTINA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1088 ARGENTINA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1089 ARGENTINA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1090 ARGENTINA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1091 ARGENTINA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1092 ARGENTINA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1093 ARGENTINA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1094 ARGENTINA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1095 ARGENTINA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1096 ARGENTINA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1097 ARGENTINA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 1098 ARGENTINA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1099 ARGENTINA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1100 ARGENTINA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 1101 ARGENTINA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1102 ARGENTINA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1103 ARGENTINA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1104 ARGENTINA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 1105 ARGENTINA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1106 ARGENTINA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 1107 ARGENTINA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 1108 ARGENTINA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 1109 CHILE STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1110 CHILE ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1111 CHILE HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1112 CHILE STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 1113 CHILE TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1114 CHILE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1115 CHILE BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1116 CHILE ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1117 CHILE THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1118 CHILE CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1119 CHILE BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1120 CHILE ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1121 CHILE ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1122 CHILE ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1123 CHILE TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1124 CHILE STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1125 CHILE SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1126 CHILE MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1127 CHILE BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1128 CHILE MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 1129 CHILE ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1130 CHILE PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1131 CHILE MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 1132 CHILE MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1133 CHILE SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1134 CHILE OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1135 CHILE DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 1136 CHILE IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1137 CHILE STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 1138 CHILE STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 1139 CHILE STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 1140 PERU STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1141 PERU ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1142 PERU HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1143 PERU STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 1144 PERU TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1145 PERU MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1146 PERU BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1147 PERU ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1148 PERU THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1149 PERU CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1150 PERU BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1151 PERU ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1152 PERU ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1153 PERU ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1154 PERU TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1155 PERU STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1156 PERU SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1157 PERU MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1158 PERU BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1159 PERU MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 1160 PERU ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1161 PERU PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1162 PERU MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 1163 PERU MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1164 PERU SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1165 PERU OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1166 PERU DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 1167 PERU IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1168 PERU STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 1169 PERU STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 1170 PERU STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 1171 REST OF SOUTH AMERICA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1172 MIDDLE EAST AND AFRICA STROKE MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 1173 MIDDLE EAST AND AFRICA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1174 MIDDLE EAST AND AFRICA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1175 MIDDLE EAST AND AFRICA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1176 MIDDLE EAST AND AFRICA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 1177 MIDDLE EAST AND AFRICA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1178 MIDDLE EAST AND AFRICA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1179 MIDDLE EAST AND AFRICA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1180 MIDDLE EAST AND AFRICA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1181 MIDDLE EAST AND AFRICA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1182 MIDDLE EAST AND AFRICA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1183 MIDDLE EAST AND AFRICA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1184 MIDDLE EAST AND AFRICA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1185 MIDDLE EAST AND AFRICA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1186 MIDDLE EAST AND AFRICA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1187 MIDDLE EAST AND AFRICA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1188 MIDDLE EAST AND AFRICA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1189 MIDDLE EAST AND AFRICA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1190 MIDDLE EAST AND AFRICA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1191 MIDDLE EAST AND AFRICA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1192 MIDDLE EAST AND AFRICA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 1193 MIDDLE EAST AND AFRICA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1194 MIDDLE EAST AND AFRICA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1195 MIDDLE EAST AND AFRICA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 1196 MIDDLE EAST AND AFRICA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1197 MIDDLE EAST AND AFRICA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1198 MIDDLE EAST AND AFRICA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1199 MIDDLE EAST AND AFRICA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 1200 MIDDLE EAST AND AFRICA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1201 MIDDLE EAST AND AFRICA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 1202 MIDDLE EAST AND AFRICA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 1203 MIDDLE EAST AND AFRICA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 1204 SOUTH AFRICA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1205 SOUTH AFRICA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1206 SOUTH AFRICA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1207 SOUTH AFRICA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 1208 SOUTH AFRICA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1209 SOUTH AFRICA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1210 SOUTH AFRICA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1211 SOUTH AFRICA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1212 SOUTH AFRICA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1213 SOUTH AFRICA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1214 SOUTH AFRICA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1215 SOUTH AFRICA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1216 SOUTH AFRICA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1217 SOUTH AFRICA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1218 SOUTH AFRICA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1219 SOUTH AFRICA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1220 SOUTH AFRICA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1221 SOUTH AFRICA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1222 SOUTH AFRICA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1223 SOUTH AFRICA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 1224 SOUTH AFRICA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1225 SOUTH AFRICA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1226 SOUTH AFRICA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 1227 SOUTH AFRICA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1228 SOUTH AFRICA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1229 SOUTH AFRICA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1230 SOUTH AFRICA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 1231 SOUTH AFRICA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1232 SOUTH AFRICA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 1233 SOUTH AFRICA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 1234 SOUTH AFRICA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 1235 SAUDI ARABIA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1236 SAUDI ARABIA ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1237 SAUDI ARABIA HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1238 SAUDI ARABIA STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 1239 SAUDI ARABIA TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1240 SAUDI ARABIA MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1241 SAUDI ARABIA BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1242 SAUDI ARABIA ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1243 SAUDI ARABIA THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1244 SAUDI ARABIA CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1245 SAUDI ARABIA BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1246 SAUDI ARABIA ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1247 SAUDI ARABIA ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1248 SAUDI ARABIA ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1249 SAUDI ARABIA TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1250 SAUDI ARABIA STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1251 SAUDI ARABIA SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1252 SAUDI ARABIA MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1253 SAUDI ARABIA BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1254 SAUDI ARABIA MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 1255 SAUDI ARABIA ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1256 SAUDI ARABIA PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1257 SAUDI ARABIA MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 1258 SAUDI ARABIA MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1259 SAUDI ARABIA SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1260 SAUDI ARABIA OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1261 SAUDI ARABIA DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 1262 SAUDI ARABIA IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1263 SAUDI ARABIA STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 1264 SAUDI ARABIA STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 1265 SAUDI ARABIA STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 1266 EGYPT STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1267 EGYPT ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1268 EGYPT HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1269 EGYPT STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 1270 EGYPT TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1271 EGYPT MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1272 EGYPT BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1273 EGYPT ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1274 EGYPT THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1275 EGYPT CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1276 EGYPT BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1277 EGYPT ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1278 EGYPT ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1279 EGYPT ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1280 EGYPT TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1281 EGYPT STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1282 EGYPT SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1283 EGYPT MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1284 EGYPT BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1285 EGYPT MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 1286 EGYPT ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1287 EGYPT PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1288 EGYPT MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 1289 EGYPT MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1290 EGYPT SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1291 EGYPT OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1292 EGYPT DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 1293 EGYPT IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1294 EGYPT STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 1295 EGYPT STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 1296 EGYPT STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 1297 U.A.E. STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1298 U.A.E. ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1299 U.A.E. HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1300 U.A.E. STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 1301 U.A.E. TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1302 U.A.E. MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1303 U.A.E. BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1304 U.A.E. ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1305 U.A.E. THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1306 U.A.E. CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1307 U.A.E. BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1308 U.A.E. ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1309 U.A.E. ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1310 U.A.E. ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1311 U.A.E. TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1312 U.A.E. STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1313 U.A.E. SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1314 U.A.E. MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1315 U.A.E. BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1316 U.A.E. MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 1317 U.A.E. ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1318 U.A.E. PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1319 U.A.E. MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 1320 U.A.E. MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1321 U.A.E. SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1322 U.A.E. OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1323 U.A.E. DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 1324 U.A.E. IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1325 U.A.E. STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 1326 U.A.E. STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 1327 U.A.E. STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 1328 ISRAEL STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1329 ISRAEL ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1330 ISRAEL HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1331 ISRAEL STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 1332 ISRAEL TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1333 ISRAEL MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1334 ISRAEL BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1335 ISRAEL ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1336 ISRAEL THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1337 ISRAEL CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1338 ISRAEL BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1339 ISRAEL ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1340 ISRAEL ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1341 ISRAEL ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1342 ISRAEL TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1343 ISRAEL STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1344 ISRAEL SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1345 ISRAEL MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1346 ISRAEL BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1347 ISRAEL MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 1348 ISRAEL ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1349 ISRAEL PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1350 ISRAEL MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 1351 ISRAEL MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1352 ISRAEL SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1353 ISRAEL OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1354 ISRAEL DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 1355 ISRAEL IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1356 ISRAEL STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 1357 ISRAEL STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 1358 ISRAEL STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 1359 KUWAIT STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1360 KUWAIT ISCHEMIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1361 KUWAIT HEMORRHAGIC STROKE IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1362 KUWAIT STROKE MARKET, BY DIAGNOSIS AND TREATMENT, 2018-2032 (USD THOUSAND)
TABLE 1363 KUWAIT TREATMENT IN STROKE MARKET, BY TREATMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1364 KUWAIT MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1365 KUWAIT BLOOD PRESSURE MEDICINE IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1366 KUWAIT ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1367 KUWAIT THIAZIDE DIURETICS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1368 KUWAIT CALCIUM CHANNEL BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1369 KUWAIT BETA BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1370 KUWAIT ALPHA-BLOCKERS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1371 KUWAIT ANTIPLATELET DRUGS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1372 KUWAIT ANTICOAGULANTS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1373 KUWAIT TISSUE PLASMINOGEN ACTIVATOR (TPA) IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1374 KUWAIT STATINS IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1375 KUWAIT SUPPORTIVE MEDICATION IN STROKE MARKET, BY CLASS, 2018-2032 (USD THOUSAND)
TABLE 1376 KUWAIT MEDICATION IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1377 KUWAIT BRANDED IN STROKE MARKET, BY DRUG TYPE, 2018-2032 (USD THOUSAND)
TABLE 1378 KUWAIT MEDICATION IN STROKE MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD THOUSAND)
TABLE 1379 KUWAIT ORAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1380 KUWAIT PARENTERAL IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1381 KUWAIT MEDICATION IN STROKE MARKET, BY MODE OF PURCHASE, 2018-2032 (USD THOUSAND)
TABLE 1382 KUWAIT MEDICATION IN STROKE MARKET, BY THERAPY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1383 KUWAIT SURGERY IN STROKE MARKET, BY INSTRUMENT TYPE, 2018-2032 (USD THOUSAND)
TABLE 1384 KUWAIT OTHER THERAPY IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1385 KUWAIT DIAGNOSIS IN STROKE MARKET, BY DIAGNOSIS TYPE, 2018-2032 (USD THOUSAND)
TABLE 1386 KUWAIT IMAGING TEST IN STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 1387 KUWAIT STROKE MARKET, BY GENDER, 2018-2032 (USD THOUSAND)
TABLE 1388 KUWAIT STROKE MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 1389 KUWAIT STROKE MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 1390 REST OF MIDDLE EAST AND AFRICA STROKE MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
Lista de figuras
FIGURE 1 GLOBAL STROKE MARKET: SEGMENTATION
FIGURE 2 GLOBAL STROKE MARKET: DATA TRIANGULATION
FIGURE 3 GLOBAL STROKE MARKET: DROC ANALYSIS
FIGURE 4 GLOBAL STROKE MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 GLOBAL STROKE MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 GLOBAL STROKE MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 GLOBAL STROKE MARKET: DBMR MARKET POSITION GRID
FIGURE 8 GLOBAL STROKE MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 GLOBAL STROKE MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 GLOBAL STROKE MARKET: SEGMENTATION
FIGURE 11 RISING INCIDENCES OF STROKE DRIVE DEMAND FOR TREATMENTS IS EXPECTED TO DRIVE THE GLOBAL STROKE MARKET GROWTH IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 12 ISCHEMIC STROKE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE GLOBAL STROKE MARKET IN THE FORECAST PERIOD OF 2025 & 2032
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE GLOBAL STROKE MARKET, AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 14 ASIA-PACIFIC IS THE FASTEST-GROWING MARKET FOR GLOBAL STROKE MARKET IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 15 GLOBAL STROKE MARKET EXECUTIVE SUMMARY
FIGURE 16 STRATEGIC DECISIONS
FIGURE 17 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE GLOBAL STROKE MARKET
FIGURE 18 GLOBAL STROKE MARKET: BY TYPE, 2024
FIGURE 19 GLOBAL STROKE MARKET: BY TYPE, 2025-2032 (USD THOUSAND)
FIGURE 20 GLOBAL STROKE MARKET: BY TYPE, CAGR (2025-2032)
FIGURE 21 GLOBAL STROKE MARKET: BY TYPE, LIFELINE CURVE
FIGURE 22 GLOBAL STROKE MARKET: BY GENDER, 2024
FIGURE 23 GLOBAL STROKE MARKET: BY GENDER, 2025 TO 2032 (USD THOUSAND)
FIGURE 24 GLOBAL STROKE MARKET: BY GENDER, CAGR (2025- 2032)
FIGURE 25 GLOBAL STROKE MARKET: BY GENDER, LIFELINE CURVE
FIGURE 26 GLOBAL STROKE MARKET: BY DIAGNOSIS AND TREATMENT, 2024
FIGURE 27 GLOBAL STROKE MARKET: BY DIAGNOSIS AND TREATMENT, 2025-2032 (USD THOUSAND)
FIGURE 28 GLOBAL STROKE MARKET: BY DIAGNOSIS AND TREATMENT, CAGR (2025-2032)
FIGURE 29 GLOBAL STROKE MARKET: BY DIAGNOSIS AND TREATMENT, LIFELINE CURVE
FIGURE 30 GLOBAL STROKE MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 31 GLOBAL STROKE MARKET: BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
FIGURE 32 GLOBAL STROKE MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025-2032)
FIGURE 33 GLOBAL STROKE MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 34 GLOBAL STROKE MARKET: BY END USER, 2024
FIGURE 35 GLOBAL STROKE MARKET: BY END USER, 2025-2032 (USD THOUSAND)
FIGURE 36 GLOBAL STROKE MARKET: BY END USER, CAGR (2025-2032)
FIGURE 37 GLOBAL STROKE MARKET: BY END USER, LIFELINE CURVE
FIGURE 38 GLOBAL STROKE MARKET: SNAPSHOT (2024)
FIGURE 39 GLOBAL STROKE MARKET: COMPANY SHARE 2024 (%)
FIGURE 40 NORTH AMERICA STROKE MARKET: COMPANY SHARE 2024 (%)
FIGURE 41 EUROPE STROKE MARKET: COMPANY SHARE 2024 (%)
FIGURE 42 ASIA-PACIFIC STROKE MARKET: COMPANY SHARE 2024 (%)
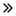
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.



















